electronic configuration of iron|Iba pa : Bacolod The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write . Lebron and his wife designed and donated furniture, replaced roofs, fixed the children’s work place and donated over 1000 computers to the club. Additionally, Lebron has used his platform to speak out about race relations and the police in the USA. . Her foundation, the Ronda Rousey Charity works with foundations such as Didi Hirsch, which .How much does a good gaming monitor cost? While you could easily spend more than $1,000 to obtain the best gaming monitor on the market now, the reality is that the budget and midrange categories .
PH0 · potassium electron configuration full
PH1 · iron 2+ electron configuration
PH2 · electronic configuration of nickel
PH3 · electron configuration of all elements
PH4 · electron configuration for fe+3
PH5 · electron configuration chart
PH6 · complete electron configuration for chlorine
PH7 · abbreviated electron configuration for iron
PH8 · Iba pa
1,535 vivian velez pinay scandal FREE videos found on XVIDEOS for this search. Language: Your location: USA Straight. Login Join for FREE Premium. Best Videos; Categories. Porn in your language; 3d; AI; . Pinay Sex Scandal First Time ni Misis nakatikim ng Malaking Burat ni Kumpare, Simot Sarap ang Tamod 8 min. 8 min Pinay .
electronic configuration of iron*******The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .
Iba paLearn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, .
electronic configuration of ironLearn how to write the electronic configuration of iron and its oxidation states, valency, and applications. Find out the reduced form of iron and the difference between iron and . The electron configuration of iron is 3d 6 4s 2, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration .
Learn how to write the electronic configuration of iron and its ions, and the valency of iron in different oxidation states. Find out the properties, allotropic forms and .
The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, . Learn how to write the electronic configuration of iron, its valency, oxidation states, properties and applications. Find out the formula of rust, the role of iron in .electronic configuration of iron Iba pa Learn how to write the electron configuration of iron using the noble gas shortcut and the energy levels of the orbitals. See the answer, the long version, and the explanation with diagrams.
Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic table is a tabular display of the chemical .
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram . Iron is a good example. Humans have known about iron long before we knew about elements and atoms. . The electron configuration is 4s 1, 3d 10 but all these general .
Fe (Iron) is an element with position number 26 in the periodic table. Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm 3 . Electronic configuration of the Iron atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6Electronic configuration of the Iron atom in ascending order of the levels: 1s 2 2s 2 .
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the .
Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic . The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, the Iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration.
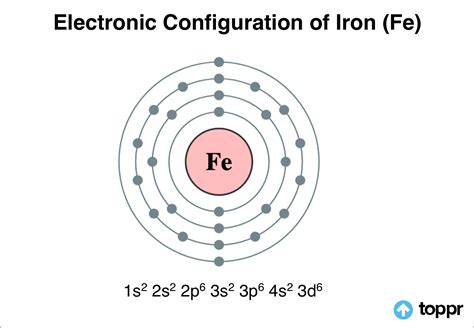
The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen.Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
Element Iron (Fe), Group 8, Atomic Number 26, d-block, Mass 55.845. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
Here are the changes in the electronic structure of iron to make the 2+ or the 3+ ion. Fe [Ar] 3d 6 4s 2: Fe 2 + [Ar] 3d 6: Fe 3 + [Ar] 3d 5: The 4s orbital and the 3d orbitals have very similar energies. There is not a huge jump in the amount of energy you need to remove the third electron compared with the first and second.The atomic number of iron is 26. So, the electronic configuration of Fe is as follows: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. Fe is a neutral atom, and the oxidation number of Fe in Fe2+ is +2 which means Fe loses 2 electrons in the process. Upon losing two electrons, it forms Fe2+. Therefore, the electronic configuration of Fe2+ is as follows: 1s . The electronic configuration of iron will be reduced to [Ar]3d6. And the Oxidation state of iron becomes +2. Iron will attain more stability by the loss of one more electron from the d subshell and thereby changing the electronic configuration to [Ar]3d5. This has more stability than iron in the +2-oxidation state and is because of the reason .The electron configuration of manganese is 1s2 2s2 2p6 3s2 3p6 3d5 4s2. Manganese is the chemical element of the periodic table of elements located in group 7, its symbol is Mn and its atomic number is 25. It is found in nature as a free element, often combined with iron and other minerals. As a free element, manganese is characterized by being .
The ground state electronic structure of the terminal form of this complex is an open-shell singlet, with two antiferromagnetically coupled electrons residing on the iron center and carbene ligand. Electronic Configuration of Iron. The shorthand electronic configuration of iron (Fe), which has an atomic number of 26, is \( [Ar] 3d^6 4s^2 \). Its electronic configuration can be written as \( 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 \). It indicates that it has six electrons in the 3d orbital and two electrons in its outermost 4s orbital.
For example, the electron configuration of iron is Fe is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated electron configuration (or noble gas configuration) to identify valence . The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the ground state electron configuration of an element. Iron (Fe) is a transition metal that follows the Aufbau rule .
The Lions are 36-17 ATS under coach Dan Campbell, the best record in the NFL in that span (since 2021). They are 21-10 ATS as underdogs and 17-9 ATS on the road under Campbell.
electronic configuration of iron|Iba pa